What is 14C ?
There are 7 isotopes of carbon: two of them are stable, 12C et 13C which constitute ca. 99% and 1%, respectively, of total carbon present on earth. The other isotopes, which occur in trace amounts, are radioactive: they decay with time.
Radioactive 14C is produced in the upper layers of the atmosphere, from cosmic ray impacts on nitrogen. The 14C concentration in the atmosphere results from a balance between production and disintegration.
Living beings absorb carbon through exchanges with their environment, so their 14C content equals the 14C content in the atmosphere. But once they die the natural 14C decay is not compensated anymore by new uptakes: the amount of 14C gradually decreases, following an exponential decay with a half-life of 5726 years. This means that the 14C concentration in a plant that died 5726 years ago is only 50% of its initial concentration; after another 5726 years, it will be the half of 50% thus 25% of the initial concentration, etc.
Dating principle
If the 14C concentration is considered constant in time and space, then fossilized organic matters may be investigated to yield the date of their death and thus of their incorporation in the deposit, on the basis of the measurement of residual 14C. This method allows to date samples (age in radiocarbon years) of maximum 50,000 years. It is applicable to wood, peat, charcoal, paper, hair, bone,... samples.
The radiocarbon age
The result of a radiocarbon measurement (made by a specialized laboratory) is reported as follows :
date ± σ BP (example : 2530 ± 50 BP)
where BP = before present; "present" is fixed by convention at 01.01.1950
σ = standard deviation.
A dating always indicates a confidence interval due to uncertainty; the probability is :
- 68% that radiocarbon age is between date-σ et date+σ;
- 95% that radiocarbon age is between date-2σ et date+2σ;
- 99% that radiocarbon age is between date-3σ et date+3σ.
The calibrated age
Actually the 14C concentration is not constant in time and space (variations in solar activity, variations in exchanges between the atmosphere and carbon reservoirs such as oceans,...).
Variation in 14C concentration in the atmosphere since 11,000 years ago:

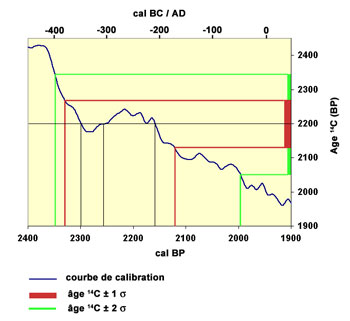
Hence, the raw 14C age computed from the exponential decay has to be calibrated to give a calendar date. Calibration curves are plotted on the basis of correlations with other dating techniques. On such curves solar ages are plotted against the corresponding 14C dates. Calibrated dates are reported with a 'cal' mention. Example : 2530 cal. BP
Calibrated dates are often expressed as :
cal. BC = before Christus cal.
AD = Anno Domini (after Christus)
The confidence interval is also recomputed in solar years.
Example :
2200 ± 70 BP >>>> between cal BP 2350 and BP 2000 (confidence interval of 95%)
or between cal BC 400 and BC 50 (confidence interval of 95%)